De-Novo RNA Sequencing Pipeline¶
RNA Sequencing is a technique that allows transcriptome studies based on high throughput next-generation gene sequencing (NGS). De novo sequencing refers to sequencing a novel genome where there is no reference sequence available for alignment. Sequence reads are assembled as contigs, and the coverage quality of de novo sequence data depends on the size and continuity of the contigs (i.e., the number of gaps in the data).
De-Novo RNASeq pipeline is adapted from the Trinity-Trinotate suggested workflow. It reconstructs transcripts from short reads, predicts proteins, and annotates, leveraging several databases. Quantification is computed using RSEM Tool, and differential expression is tested in a manner identical to the RNA-seq pipeline. We observed that the default parameters of the Trinity suite are very conservative, which could result in the loss of low-expressed but biologically relevant transcripts. To provide the most complete set of transcripts, the pipeline was designed with lower stringency during the assembly step in order to produce every possible transcript and not miss low-expressed messenger RNA. A stringent filtration step is included afterward in order to provide a set of transcripts that make sense biologically.
Introduction¶
The standard MUGQIC RNA-Seq De Novo Assembly pipeline uses the Trinity software suite to reconstruct transcriptomes from RNA-Seq data without using any reference genome or transcriptome. First, reads are trimmed with Trimmomatic and normalized in order to reduce memory requirement and decrease assembly runtime, using the Trinity normalization utility inspired by the Diginorm algorithm.
Then, the transcriptome is assembled on normalized reads using the Trinity assembler. Trinity creates a Trinity.fasta file with a list of contigs representing the transcriptome isoforms. Those transcripts are grouped in components mostly representing genes. Components and transcripts are functionally annotated using the Trinotate suite. Gene abundance estimation for each sample has been performed using RSEM Tool (RNA-Seq by Expectation-Maximization). Differential gene expression analysis is performed using DESeq2 and edgeR Bioconductor packages.
The DESeq2 and edgeR methods model count data by a negative binomial distribution. The parameters of the distribution (mean and dispersion) are estimated from the data, i.e. from the read counts in the input files. Both methods compute a measure of read abundance, i.e. expression level (called base mean or mean of normalized counts in DESeq2, and concentration in edgeR) for each gene and apply a hypothesis test to each gene to evaluate differential expression. In particular, both methods determine a p-value and a log2 fold change (in expression level) for each gene. The Log2 FC of edgeR is reported in the differential gene results file, one file per design.
The log2fold change is the logarithm (to basis 2) of the fold change condition from condition A to B (mutation or treatment are the most common conditions). A “fold change” between conditions A and B at a gene or transcript is normally computed as the ratio at gene or transcript of the base mean of scaled counts for condition B to the base mean of scaled counts for condition A. Counts are scaled by a size factor in a step called normalization (if the counts of non-differentially expressed genes in one sample are, on average, twice as high as in another, the size factor for the first sample should be twice that of the other sample). Each column of the count table is then divided by the size factor for this column and the count values are brought to a common scale, making them comparable. See the edgeR vignette for additional information on normalization approaches used in the pipeline.
The differential gene analysis is followed by a Gene Ontology (GO) enrichment analysis. This analysis use the goseq approach. The goseq is based on the use of non-native GO terms resulting from trinotate annotations (see details in the section 5 of the corresponding vignette.
Thus a high quality contigs assembly is created by extracting all transcripts having a functional annotation as defined by trinotate, the Top BLASTX hit and TmHMM annotations are used by default.
Finally, different exploratory data analysis (EDA) techniques are applied to filtered isoforms expression levels. Main goals of expression level EDA are the detection of outliers, potential mislabeling, to explore the homogeneity of biological replicates and to appreciate the global effects of the different experimental variables.
An HTML summary report is automatically generated by the pipeline. This report contains description of the sequencing experiment as well as a detailed presentation of the pipeline steps and results. Various Quality Control (QC) summary statistics are included in the report and additional QC analysis is accessible for download directly through the report. The report includes also the main references of the software and methods used during the analysis, together with the full list of parameters that have been passed to the pipeline main script.
Version¶
3.6.2
For the latest implementation and usage details refer to RNA Sequencing implementation README file file.
Usage¶
usage: rnaseq_denovo_assembly.py [-h] [--help] [-c CONFIG [CONFIG ...]]
[-s STEPS] [-o OUTPUT_DIR]
[-j {pbs,batch,daemon,slurm}] [-f]
[--no-json] [--report] [--clean]
[-l {debug,info,warning,error,critical}]
[--sanity-check]
[--container {wrapper, singularity} <IMAGE PATH>
[--genpipes_file GENPIPES_FILE]
[-d DESIGN] [-t {cufflinks,stringtie}]
[-r READSETS] [-v]
Optional Arguments
-t {cufflinks,stringtie}, --type {cufflinks,stringtie}
Type of RNA-seq Denovo assembly method
(default stringtie, faster than cufflinks)
-d DESIGN, --design DESIGN
design file
-r READSETS, --readsets READSETS
readset file
-h show this help message and exit
--help show detailed description of pipeline and steps
-c CONFIG [CONFIG ...], --config CONFIG [CONFIG ...]
config INI-style list of files; config parameters
are overwritten based on files order
-s STEPS, --steps STEPS step range e.g. '1-5', '3,6,7', '2,4-8'
-o OUTPUT_DIR, --output-dir OUTPUT_DIR
output directory (default: current)
-j {pbs,batch,daemon,slurm}, --job-scheduler {pbs,batch,daemon,slurm}
job scheduler type (default: slurm)
-f, --force force creation of jobs even if up to date (default:
false)
--no-json do not create JSON file per analysed sample to track
the analysis status (default: false i.e. JSON file
will be created)
--report create 'pandoc' command to merge all job markdown
report files in the given step range into HTML, if
they exist; if --report is set, --job-scheduler,
--force, --clean options and job up-to-date status
are ignored (default: false)
--clean create 'rm' commands for all job removable files in
the given step range, if they exist; if --clean is
set, --job-scheduler, --force options and job up-to-
date status are ignored (default: false)
-l {debug,info,warning,error,critical}, --log {debug,info,warning,error,critical}
log level (default: info)
--sanity-check run the pipeline in `sanity check mode` to verify
all the input files needed for the pipeline to run
are available on the system (default: false)
--container {wrapper, singularity} <IMAGE PATH>
run pipeline inside a container providing a container
image path or accessible singularity hub path
-v, --version show the version information and exita
-g GENPIPES_FILE, --genpipes_file GENPIPES_FILE
Commands for running the pipeline are output to this
file pathname. The data specified to pipeline command
line is processed and pipeline run commands are
stored in GENPIPES_FILE, if this option is specified
. Otherwise, the output will be redirected to stdout
. This file can be used to actually "run the
GenPipes Pipeline".
Example Run¶
Use the following commands to execute the De Novo sequencing pipeline:
rnaseq_denovo_assembly.py -c $MUGQIC_PIPELINES_HOME/pipelines/rnaseq_denovo_assembly/rnaseq_denovo_assembly.base.ini $MUGQIC_PIPELINES_HOME/pipelines/rnaseq_denovo_assembly/rnaseq_denovo_assembly.guillimin.ini -r readset.rnaseq.txt -d design.rnaseq.txt -s 1-23 -g rnaseqDeNovoCommands.sh
bash rnaseqDeNovoCommands.sh
Warning
While issuing the pipeline run command, use `-g GENPIPES_FILE` option (see example above) instead of using the ` > GENPIPES_FILE` option supported by GenPipes so far, as shown below:
[genpipes_seq_pipeline].py -t mugqic -c $MUGQIC_PIPELINES_HOME/pipelines/[genpipes_seq_pipeline]/[genpipes_seq_pipeline].base.ini $MUGQIC_PIPELINES_HOME/pipelines/[genpipes_seq_pipeline]/[genpipes_seq_pipeline].guillimin.ini -r readset.[genpipes_seq_pipeline].txt -s 1-6 > [genpipes_seq_pipeline]_commands_mugqic.sh
bash [genpipes_seq_pipeline]_commands_mugqic.sh
` > scriptfile` should be considered deprecated and `-g scriptfile` option is recommended instead.
Please note that redirecting commands to a script `> genpipe_script.sh` is still supported for now. But going forward, this mechanism might be dropped in a future GenPipes release.
You can download the test dataset for this pipeline here.
Pipeline Schema¶
Figure below shows the schema of RNA Sequencing De Novo Assembly pipeline.
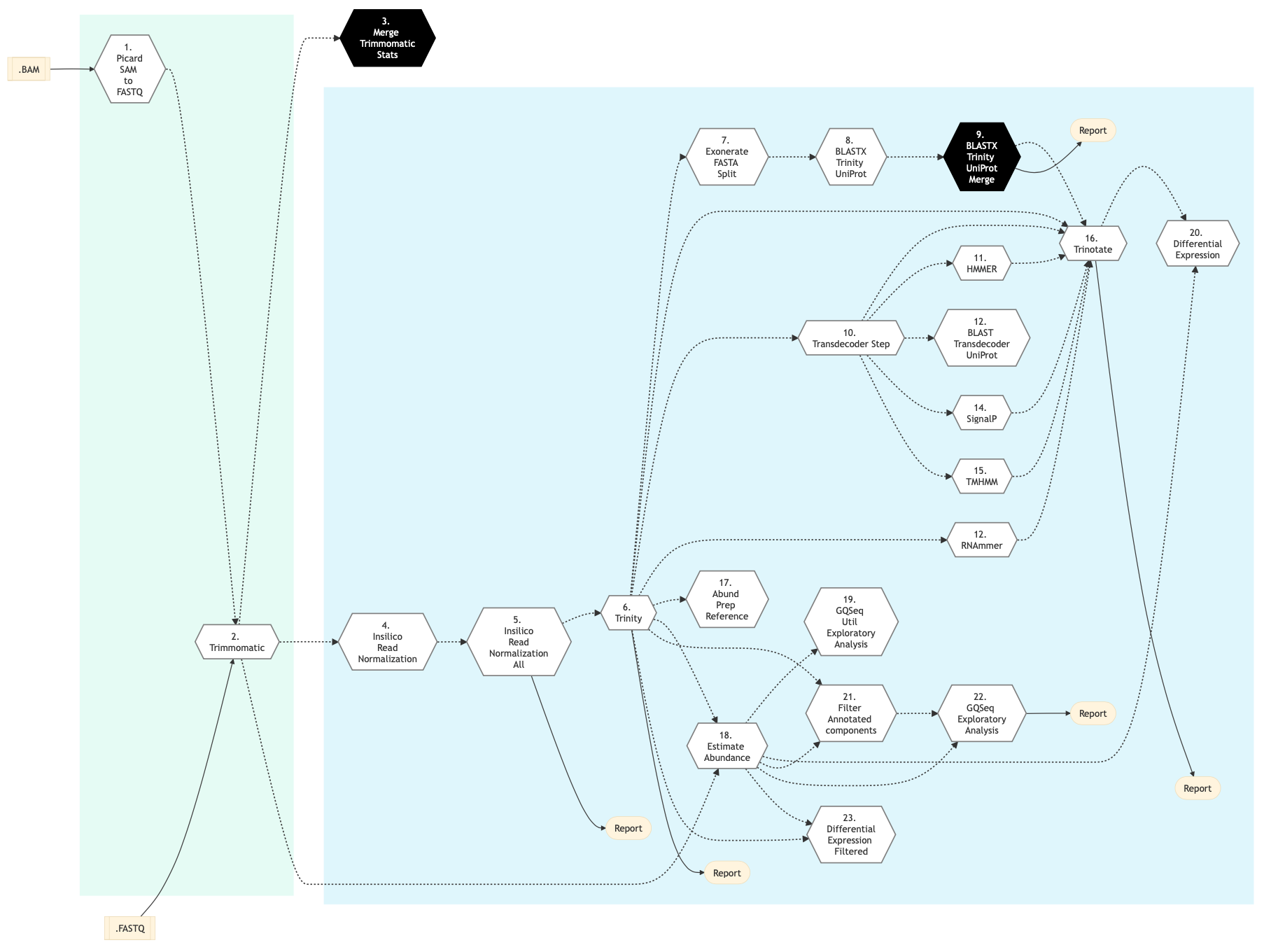
Figure: Schema of De Novo assembly RNA Sequencing protocol¶
Pipeline Steps¶
The table below lists various steps that constitute the RNA Sequencing De Novo Assembly.
RNA Sequencing De Novo Assembly Steps |
|
|---|---|
Step Details¶
Picard SAM to FastQ
Convert SAM/BAM files from the input readset file into FASTQ format if FASTQ files are not already specified in the readset file. Do nothing otherwise.
Trimmomatic
Raw reads quality trimming and removing of Illumina adapters is performed using Trimmomatic Tool. If an adapter FASTA file is specified in the config file (section ‘trimmomatic’, param ‘adapter_fasta’), it is used first. Else, ‘Adapter1’ and ‘Adapter2’ columns from the readset file are used to create an adapter FASTA file, given then to Trimmomatic. For PAIRED_END readsets, readset adapters are reversed-complemented and swapped, to match Trimmomatic Palindrome strategy. For SINGLE_END readsets, only Adapter1 is used and left unchanged.
This step takes as input files:
FASTQ files from the readset file if available
Else, FASTQ output files from previous picard_sam_to_fastq conversion of BAM files
Merge Trimmomatic Stats
The trim statistics per readset are merged at this step.
InSilico Read Normalization of Readsets
Normalize each readset, using the Trinity normalization utility.
InSilico Read Normalization (All)
Merge all normalized readsets together and normalize the result, using the Trinity normalization utility.
Trinity
Create a de novo assembly from normalized readsets using Trinity tool.
Exonerate FASTA Split
Split the Trinity assembly FASTA into chunks for further parallel BLAST annotations.
BLASTX Trinity UniProt
Annotate Trinity FASTA chunks with Swiss-Prot and UniRef databases using BLAST Tool.
BLASTX Trinity UniProt Merge
Merge blastx Swiss-Prot and UniRef chunks results.
TransDecoder Step
Identifies candidate coding regions within transcript sequences using Transdecoder.
HMMER Biosequence Analysis Step
Identifies protein domains using HMMER Biosequence Analysis
RNAmmer Method
Identify potential rRNA transcripts using RNAmmer Transcriptome.
BLAST Transdecoder UniProt
Search Transdecoder-predicted coding regions for sequence homologies on UniProt using BLASTP.
SignalP Method
Predict the presence of signal peptides and the location of their cleavage sites in proteins using SignalP.
TMHMM Method
This step involves prediction of transmembrane helices regions in proteins using TMHMM.
Trinotate
This step performs transcriptome functional annotation and analysis using Trinotate Annotation suite. ll functional annotation data is integrated into a SQLite database and a whole annotation report is created.
Align and estimate Abundance Prep Reference
Index Trinity FASTA file for further abundance estimation using Trinity align_and_estimate_abundance.pl utility.
Align and estimate Abundance
Estimate transcript abundance using RNA-Seq by Expectation-Maximization (RSEM) via Trinity align_and_estimate_abundance.pl utility.
Exploratory Analysis with gqSeqUtils R package
This step performs exploratory analysis using the gqSeqUtils R package.
Differential Expression
This step performs differential gene expression analysis using DESeq package and edgeR package. Merge the results of the analysis in a single csv file. Also,this pipeline step performs Gene Ontology analysis for RNA-Seq denovo Assembly using the Bioconductor’s R package goseq. It generates GO annotations for differential genes and isoforms expression analysis, based on associated GOTERMS generated by Trinotate Step earlier.
Filter Annotated Components
This step filters high quality contigs based on values in trinotate annotations. It also recreates a high quality contigs fasta file and run Assembly statistics using the gqSeqUtils R package.
Exploratory Analysis with subset of filtered transcripts
In this step, exploratory analysis is performed by the gqSeqUtils R package using a subset of filtered transcripts.
GOSEQ using filtered transcripts
In this step, differential expression and goseq analysis is performed based on filtered transcripts and genes.
More information¶
You can find more information about RNA Sequencing De Novo Assembly Pipeline in the following references:
Grabherr MG, Haas BJ, Yassour M, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome - Trinity-Trinotate.
Chin CS, Alexander DH, Marks P, et al. Non-hybrid, finished microbial genome assemblies from long-read SMRT sequencing data - suggested workflow.
Trinity RNA sequencing utilities Workshop Slides.